Food Allergies and Thanksgiving
November 12, 2025 | Black & Kletz Allergy
 Well, it is that time of year again. A time for getting together with family and friends for the annual ritual of gluttony - I mean Thanksgiving. Although the holiday is generally a festive occasion, some food-allergic individuals find Thanksgiving to be a time of the year where they are more concerned than usual. Individuals with food allergies are always concerned when eating at a restaurant or at someone’s house due to the fear of consuming food that causes them to have food allergy symptoms. On Thanksgiving, however, this fear is intensified because typically the food offered on Thanksgiving at someone’s home is usually made by many different people. Normally, when it is not Thanksgiving, the allergic individual can tell the host/hostess what food allergies they have, and the host/hostess will avoid serving that food or foods. On Thanksgiving however, since there are usually multiple people either preparing or cooking the meal, there is more of a chance that an allergic ingredient will be used simply because the person making the food either was not told about the food allergy, they forgot to avoid using it, or it was not communicated to them about the food allergy by the host/hostess. As a result, more mistakes may be made, and a person is more likely to eat food that he or she is allergic to than in other more intimate gatherings.
Common food allergens such as milk, egg, wheat, soy, peanuts, and tree nuts are often found around the Thanksgiving table. Gravy, used for turkey and mashed potatoes, frequently contains the food allergens dairy, soy, and/or wheat. Tree nuts (e.g., almonds) are frequently found on string beans and in some types of stuffing (e.g., chestnuts). Tree nuts and peanuts are common in many desserts such as brownies and pecan pie. Milk (i.e., dairy) and eggs are also used in many baked goods. Although pumpkin allergies are not common, pumpkin pie may contain a host of ingredients that may induce a food allergy in some sensitive individuals. It is significant to note that among various cultures, many families incorporate many ethnic foods in their celebrations. These foods may not be traditional, but they may increase the probability of other allergenic foods such as shellfish, fish, etc. to be the cause of an imminent food allergy. If a person has a serious food allergy, it is sensible for that individual to bring their own food, so they can avoid any potential allergic reaction from food.
Although having an allergy to turkey does exit, it is not a common food allergy. It is worth noting however that many people feel sleepy after consuming turkey, particularly on Thanksgiving when large amounts are typically eaten. The reason for the sleepiness may stem from the fact that turkey contains higher levels of the amino acid “L-tryptophan.” The amino acid L-tryptophan is an essential amino acid that has to be obtained from foods we eat since the body does not make L-tryptophan on its own. Amino acids are the precursors to proteins and it takes many amino acids to create a protein. Thus, amino acids are considered to be the “building blocks” of proteins. It is also important to mention that there are many other foods besides turkey that are rich in L-tryptophan. Some of these foods include oats, eggs, canned tuna, fish, legumes (e.g., soybeans, peanuts, lima beans), milk, cheese, yogurt, seeds (e.g., pumpkin, sunflower, sesame), bread, chocolate, and some fruits. It should be stated that turkey and chicken both have comparable amounts of L-tryptophan. Another thought-provoking fact is that the dark turkey meat has less of the amino acid L-tryptophan than the white turkey meat, but with chicken, it is the opposite, as there is less L-tryptophan in white chicken meat than dark chicken meat. After eating turkey, the L-tryptophan will enter one’s bloodstream from the digestive tract and travel to the brain where it gets converted to the chemical “serotonin.” It is the serotonin that is responsible for causing the sleepiness associated with eating turkey. It should also be pointed out that eating a high carbohydrate, high fat meal can also lead to sleepiness and fatigue about 1-2 hours after eating. Serotonin, however, is a chemical in the brain that plays a role in one’s mood, as well as pain intolerance. High serotonin levels are associated with elevated moods and a sense of relaxation. Serotonin may also increase one’s pain tolerance. In addition, L-tryptophan is thought to have a beneficial effect on learning, memory, and depression. Serotonin may also have useful effects on decreasing anxiety, premenstrual pain, and seasonal affective disorder although more research is needed to verify this. So, if you experience sleepiness at the Thanksgiving Day table, it may not be your in-laws at all, it may be the turkey! Likewise, if you are in a good mood and feel less depressed during Thanksgiving, it may be that you are happy to be with family, but it still could be the turkey!
The board certified allergists at Black & Kletz Allergy diagnose and treat both adult and pediatric patients. We have offices in Washington, DC, McLean, VA (Tysons Corner, VA), and Manassas, VA. All 3 of our offices have on-site parking. The Washington, DC and McLean, VA offices are Metro accessible and the McLean, VA office has a free shuttle that runs between our office and the Spring Hill metro station on the silver line. You may also click Request an Appointment and we will respond within 24 hours by the next business day. Black & Kletz Allergy has been a fixture in the greater Washington, DC, Northern Virginia, and Maryland metropolitan community for over 50 years for our outstanding services for the diagnosis and treatment of allergic, asthmatic, and immunological conditions.
Well, it is that time of year again. A time for getting together with family and friends for the annual ritual of gluttony - I mean Thanksgiving. Although the holiday is generally a festive occasion, some food-allergic individuals find Thanksgiving to be a time of the year where they are more concerned than usual. Individuals with food allergies are always concerned when eating at a restaurant or at someone’s house due to the fear of consuming food that causes them to have food allergy symptoms. On Thanksgiving, however, this fear is intensified because typically the food offered on Thanksgiving at someone’s home is usually made by many different people. Normally, when it is not Thanksgiving, the allergic individual can tell the host/hostess what food allergies they have, and the host/hostess will avoid serving that food or foods. On Thanksgiving however, since there are usually multiple people either preparing or cooking the meal, there is more of a chance that an allergic ingredient will be used simply because the person making the food either was not told about the food allergy, they forgot to avoid using it, or it was not communicated to them about the food allergy by the host/hostess. As a result, more mistakes may be made, and a person is more likely to eat food that he or she is allergic to than in other more intimate gatherings.
Common food allergens such as milk, egg, wheat, soy, peanuts, and tree nuts are often found around the Thanksgiving table. Gravy, used for turkey and mashed potatoes, frequently contains the food allergens dairy, soy, and/or wheat. Tree nuts (e.g., almonds) are frequently found on string beans and in some types of stuffing (e.g., chestnuts). Tree nuts and peanuts are common in many desserts such as brownies and pecan pie. Milk (i.e., dairy) and eggs are also used in many baked goods. Although pumpkin allergies are not common, pumpkin pie may contain a host of ingredients that may induce a food allergy in some sensitive individuals. It is significant to note that among various cultures, many families incorporate many ethnic foods in their celebrations. These foods may not be traditional, but they may increase the probability of other allergenic foods such as shellfish, fish, etc. to be the cause of an imminent food allergy. If a person has a serious food allergy, it is sensible for that individual to bring their own food, so they can avoid any potential allergic reaction from food.
Although having an allergy to turkey does exit, it is not a common food allergy. It is worth noting however that many people feel sleepy after consuming turkey, particularly on Thanksgiving when large amounts are typically eaten. The reason for the sleepiness may stem from the fact that turkey contains higher levels of the amino acid “L-tryptophan.” The amino acid L-tryptophan is an essential amino acid that has to be obtained from foods we eat since the body does not make L-tryptophan on its own. Amino acids are the precursors to proteins and it takes many amino acids to create a protein. Thus, amino acids are considered to be the “building blocks” of proteins. It is also important to mention that there are many other foods besides turkey that are rich in L-tryptophan. Some of these foods include oats, eggs, canned tuna, fish, legumes (e.g., soybeans, peanuts, lima beans), milk, cheese, yogurt, seeds (e.g., pumpkin, sunflower, sesame), bread, chocolate, and some fruits. It should be stated that turkey and chicken both have comparable amounts of L-tryptophan. Another thought-provoking fact is that the dark turkey meat has less of the amino acid L-tryptophan than the white turkey meat, but with chicken, it is the opposite, as there is less L-tryptophan in white chicken meat than dark chicken meat. After eating turkey, the L-tryptophan will enter one’s bloodstream from the digestive tract and travel to the brain where it gets converted to the chemical “serotonin.” It is the serotonin that is responsible for causing the sleepiness associated with eating turkey. It should also be pointed out that eating a high carbohydrate, high fat meal can also lead to sleepiness and fatigue about 1-2 hours after eating. Serotonin, however, is a chemical in the brain that plays a role in one’s mood, as well as pain intolerance. High serotonin levels are associated with elevated moods and a sense of relaxation. Serotonin may also increase one’s pain tolerance. In addition, L-tryptophan is thought to have a beneficial effect on learning, memory, and depression. Serotonin may also have useful effects on decreasing anxiety, premenstrual pain, and seasonal affective disorder although more research is needed to verify this. So, if you experience sleepiness at the Thanksgiving Day table, it may not be your in-laws at all, it may be the turkey! Likewise, if you are in a good mood and feel less depressed during Thanksgiving, it may be that you are happy to be with family, but it still could be the turkey!
The board certified allergists at Black & Kletz Allergy diagnose and treat both adult and pediatric patients. We have offices in Washington, DC, McLean, VA (Tysons Corner, VA), and Manassas, VA. All 3 of our offices have on-site parking. The Washington, DC and McLean, VA offices are Metro accessible and the McLean, VA office has a free shuttle that runs between our office and the Spring Hill metro station on the silver line. You may also click Request an Appointment and we will respond within 24 hours by the next business day. Black & Kletz Allergy has been a fixture in the greater Washington, DC, Northern Virginia, and Maryland metropolitan community for over 50 years for our outstanding services for the diagnosis and treatment of allergic, asthmatic, and immunological conditions.
Rhapsido – A New Treatment for Chronic Urticaria (Hives)
October 23, 2025 | Black & Kletz Allergy
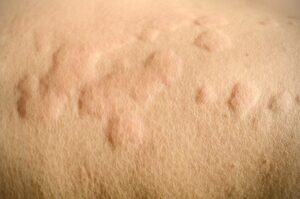 Urticaria (i.e., hives) are itchy red blotches or “welts” that generally occur on the skin but may also occur internally on various parts of the body. The lesions can be flush with the skin, but are usually slightly elevated above the skin surface, similar to a mosquito bite. Approximately 20 to 30% of the population will develop hives at some point during their lifetime.
Individual skin lesions typically subside in less than 24 hours, although an individual may have multiple crops of lesions in a single day. In some individuals however, complex hives caused by the inflammation of blood vessels in the skin may last more than 24 hours and leave residual marks on the skin after their resolution.
In a majority of instances, the episodes of hives subside spontaneously within a few weeks, however, if the condition lasts longer than 6 weeks, it is termed chronic urticaria. Alternatively, if the hives last shorter than 6 weeks in duration, the condition is referred to as acute urticaria. Sometimes hives are associated with the swelling of soft tissues (i.e., angioedema) commonly involving the eyelids, lips, throat, and/or tongue. It should be noted that angioedema can occur anywhere on or in the body as they can also appear internally.
The itching (i.e., pruritus) severity can run the spectrum from very mild to very severe and may be present both during day and/or night. It can impact one’s quality of sleep at night, impair one’s concentration in school or work during the daytime, thus adversely impacting one’s overall quality of life.
Common triggers for hives may include a side effect of a medication, allergic sensitivity to foods, and/or viral infections. In the vast majority of cases however, there will be no identifiable triggering factor(s) and as a result, it is referred to as chronic spontaneous urticaria (CSU). If exposure to cold, heat, water, vibration, or pressure triggers hives, it is characterized as chronic inducible urticaria (CIU).
A thorough and comprehensive history of the onset, duration, severity, and exposure to possible triggers may provide clues to the causation of the condition. A few screening laboratory tests are usually obtained in order to rule out systemic disorders which could manifest as chronic spontaneous urticaria. In most instances (approximately 95%) however, the screening tests will be normal an no identifiable cause will be found.
Although no cause can be identified in most cases, the treatment of hives is usually quite manageable. Medications can be very helpful in relieving the bothersome itching that is often associated with hives. Medications are also used to lessen the frequency and severity of the actual hives themselves. Histamine, a chemical released into the blood stream from both mast cells and basophils (i.e., types of white blood cells) is the principle chemical mediator that causes the clinical manifestations of both acute and chronic urticaria.
Medications which block the actions of histamine on the skin are usually first line treatment options. Second-generation antihistamines such as Claritin (i.e., loratadine), Allegra (i.e., fexofenadine), Zyrtec (i.e., cetirizine), and Xyzal (i.e., levocetirizine) are less sedating than the first-generation antihistamines such as Benadryl (i.e., diphenhydramine) and Atarax (i.e., hydroxyzine). It should be noted that many patients do not respond adequately to the regular doses of antihistamines used in the treatment of environmental allergies. Depending on the response, the doses of these agents may be gradually increased up to 4 times the regular daily dose, if recommended by the allergist and monitored closely. If the symptoms continue to be bothersome, first-generation antihistamines such as Palgic (i.e., carbinoxamine) or Periactin (i.e., cyproheptadine) can be added to the regimen.
As we understand more and more about the underlying immunological mechanisms and the molecular mediators of these conditions, newer medications targeting these pathways and/or blocking these molecules are being developed.
When antihistamines alone are ineffective, biological medications such as Xolair (i.e., omalizumab) and Dupixent (i.e., dupilumab) can offer significant relief from symptoms. Xolair was the first biologic approved for chronic spontaneous urticaria and many patients have greatly benefited from this medication over the past several years. It is usually given as an injection under the skin (i.e., subcutaneously or SQ) every 4 weeks. Dupixent was the second biologic medication approved for chronic spontaneous urticaria in April 2025 and is administered as a subcutaneous injection (SQ) every 2 weeks.
The most recently approved medication for chronic spontaneous urticaria called Rhapsido (i.e., remibrutinib) inhibits an inflammatory mediator called Bruton’s tyrosine kinase (BTK). BTK is an intracellular protein expressed in mast cells, basophils, as well as other cells and is involved in intracellular signaling. Clinical trials have shown a 70% reduction in hives vs. a 39% decrease in hives with the placebo at week 12. It has also been shown to reduce itching by 67% vs. a decrease of 42% with the placebo.
Rhapsido got approval from the FDA in September 2025 for the treatment of chronic hives in adults with no known external triggers and who are not controlled with antihistamines. The dosage is a 25 mg pill taken by mouth twice daily. Rhapsido may be taken with or without food. It would be a good option for patients who are needle phobic or in patients who do not respond adequately to Xolair or Dupixent.
The board certified allergy specialists at Black & Kletz Allergy will promptly answer any questions you may have regarding both acute or chronic urticaria (i.e., hives) or any related condition such as generalized itching (i.e., pruritus) or swelling episodes (i.e., angioedema). Our allergy doctors have been diagnosing and treating hives in the Washington, DC, Northern Virginia, and Maryland metropolitan area for more than 50 years. We have 3 convenient locations in the Washington, DC metro area with offices in Washington, DC, McLean, VA (Tysons Corner, VA), and Manassas, VA. There is on-site parking at each location and both the Washington, DC and McLean, VA offices are Metro accessible. There is a free shuttle that runs between our McLean, VA office and the Spring Hill metro station on the silver line. To schedule an appointment, please call us at any one of our 3 locations. Alternatively, you can click Request an Appointment and we will respond within 24 hours by the next business day. Black & Kletz Allergy is dedicated in providing the most current diagnostic and treatment methods in the field of allergy, asthma, and immunology in a caring and professional setting.
Urticaria (i.e., hives) are itchy red blotches or “welts” that generally occur on the skin but may also occur internally on various parts of the body. The lesions can be flush with the skin, but are usually slightly elevated above the skin surface, similar to a mosquito bite. Approximately 20 to 30% of the population will develop hives at some point during their lifetime.
Individual skin lesions typically subside in less than 24 hours, although an individual may have multiple crops of lesions in a single day. In some individuals however, complex hives caused by the inflammation of blood vessels in the skin may last more than 24 hours and leave residual marks on the skin after their resolution.
In a majority of instances, the episodes of hives subside spontaneously within a few weeks, however, if the condition lasts longer than 6 weeks, it is termed chronic urticaria. Alternatively, if the hives last shorter than 6 weeks in duration, the condition is referred to as acute urticaria. Sometimes hives are associated with the swelling of soft tissues (i.e., angioedema) commonly involving the eyelids, lips, throat, and/or tongue. It should be noted that angioedema can occur anywhere on or in the body as they can also appear internally.
The itching (i.e., pruritus) severity can run the spectrum from very mild to very severe and may be present both during day and/or night. It can impact one’s quality of sleep at night, impair one’s concentration in school or work during the daytime, thus adversely impacting one’s overall quality of life.
Common triggers for hives may include a side effect of a medication, allergic sensitivity to foods, and/or viral infections. In the vast majority of cases however, there will be no identifiable triggering factor(s) and as a result, it is referred to as chronic spontaneous urticaria (CSU). If exposure to cold, heat, water, vibration, or pressure triggers hives, it is characterized as chronic inducible urticaria (CIU).
A thorough and comprehensive history of the onset, duration, severity, and exposure to possible triggers may provide clues to the causation of the condition. A few screening laboratory tests are usually obtained in order to rule out systemic disorders which could manifest as chronic spontaneous urticaria. In most instances (approximately 95%) however, the screening tests will be normal an no identifiable cause will be found.
Although no cause can be identified in most cases, the treatment of hives is usually quite manageable. Medications can be very helpful in relieving the bothersome itching that is often associated with hives. Medications are also used to lessen the frequency and severity of the actual hives themselves. Histamine, a chemical released into the blood stream from both mast cells and basophils (i.e., types of white blood cells) is the principle chemical mediator that causes the clinical manifestations of both acute and chronic urticaria.
Medications which block the actions of histamine on the skin are usually first line treatment options. Second-generation antihistamines such as Claritin (i.e., loratadine), Allegra (i.e., fexofenadine), Zyrtec (i.e., cetirizine), and Xyzal (i.e., levocetirizine) are less sedating than the first-generation antihistamines such as Benadryl (i.e., diphenhydramine) and Atarax (i.e., hydroxyzine). It should be noted that many patients do not respond adequately to the regular doses of antihistamines used in the treatment of environmental allergies. Depending on the response, the doses of these agents may be gradually increased up to 4 times the regular daily dose, if recommended by the allergist and monitored closely. If the symptoms continue to be bothersome, first-generation antihistamines such as Palgic (i.e., carbinoxamine) or Periactin (i.e., cyproheptadine) can be added to the regimen.
As we understand more and more about the underlying immunological mechanisms and the molecular mediators of these conditions, newer medications targeting these pathways and/or blocking these molecules are being developed.
When antihistamines alone are ineffective, biological medications such as Xolair (i.e., omalizumab) and Dupixent (i.e., dupilumab) can offer significant relief from symptoms. Xolair was the first biologic approved for chronic spontaneous urticaria and many patients have greatly benefited from this medication over the past several years. It is usually given as an injection under the skin (i.e., subcutaneously or SQ) every 4 weeks. Dupixent was the second biologic medication approved for chronic spontaneous urticaria in April 2025 and is administered as a subcutaneous injection (SQ) every 2 weeks.
The most recently approved medication for chronic spontaneous urticaria called Rhapsido (i.e., remibrutinib) inhibits an inflammatory mediator called Bruton’s tyrosine kinase (BTK). BTK is an intracellular protein expressed in mast cells, basophils, as well as other cells and is involved in intracellular signaling. Clinical trials have shown a 70% reduction in hives vs. a 39% decrease in hives with the placebo at week 12. It has also been shown to reduce itching by 67% vs. a decrease of 42% with the placebo.
Rhapsido got approval from the FDA in September 2025 for the treatment of chronic hives in adults with no known external triggers and who are not controlled with antihistamines. The dosage is a 25 mg pill taken by mouth twice daily. Rhapsido may be taken with or without food. It would be a good option for patients who are needle phobic or in patients who do not respond adequately to Xolair or Dupixent.
The board certified allergy specialists at Black & Kletz Allergy will promptly answer any questions you may have regarding both acute or chronic urticaria (i.e., hives) or any related condition such as generalized itching (i.e., pruritus) or swelling episodes (i.e., angioedema). Our allergy doctors have been diagnosing and treating hives in the Washington, DC, Northern Virginia, and Maryland metropolitan area for more than 50 years. We have 3 convenient locations in the Washington, DC metro area with offices in Washington, DC, McLean, VA (Tysons Corner, VA), and Manassas, VA. There is on-site parking at each location and both the Washington, DC and McLean, VA offices are Metro accessible. There is a free shuttle that runs between our McLean, VA office and the Spring Hill metro station on the silver line. To schedule an appointment, please call us at any one of our 3 locations. Alternatively, you can click Request an Appointment and we will respond within 24 hours by the next business day. Black & Kletz Allergy is dedicated in providing the most current diagnostic and treatment methods in the field of allergy, asthma, and immunology in a caring and professional setting.
The Association Between Asthma and GERD (Acid Reflux)
October 10, 2025 | Black & Kletz Allergy
 Individuals with asthma probably are unaware of the association between asthma and acid reflux (GERD). It is estimated that approximately 75% of asthmatics have GERD of some degree. GERD or acid reflux occurs when there is a backflow of stomach contents into the esophagus (i.e., swallowing tube). In other words, the stomach contents travel in the wrong direction and enter the esophagus, instead of the small intestines. People with GERD often complain of “heartburn,” which in reality is a burning sensation in the chest and/or throat usually in conjunction with a sour or bitter taste in the mouth. In addition, some individuals may experience other symptoms which may include sore throat, burping, abdominal bloating, nausea, wheezing, coughing, and/or the sensation that something is caught in one’s throat. It is the wheezing and coughing symptoms that cam mimic asthma in individuals without asthma. In true asthmatics, acid reflux may actually worsen their asthma symptoms, not just mimic them.
In patients with asthma, the typical asthma symptoms of chest tightness, coughing, wheezing, and/or shortness of breath may be aggravated if the underlying acid reflux is not treated appropriately. The first way this may occur is via small amounts of acid irritating the airways (similar to a chemical burn) which may in turn cause asthma symptoms. The second method may involve the triggering of a reflex in the airways to become narrower in order to prevent more acid from entering the airways. It is this narrowing of the airways that causes an individual with asthma to cough, wheeze, cough, experience chest tightness and/or feel short of breath.
In addition to the 2 methods above, some asthma medications may decrease the lower esophageal sphincter pressure thus relaxing this muscle which in turn will increase the severity of GERD or acid reflux. Asthma medications in the bronchodilator family such as albuterol (i.e., Proventil, ProAir, Ventolin, AccuNeb), terbutaline (i.e., Brethaire, Brethine), vilanterol, ipatroprium (i.e., Atrovent), salmeterol (i.e., Serevent), formoterol, (i.e., Foradil), levalbuterol (i.e., Xopenex), and Tiotropium (i.e., Spiriva) fall into this category. There are also asthma medications that are combinations of two or more medications, one of which is a bronchodilator, which can therefore increase acid reflux disease. The names of some of these medications include Advair, Dulera, Breo Ellipta, Symbicort, Combivent, AIRSUPRA, Trelegy, and DuoNeb. Theophylline (i.e., Theo-Dur, Uniphyl, Slo-Bid, Theo-24), an older but still useful oral bronchodilator asthma medication, has also been associated with an increase in GERD in patients by causing the relaxation of the lower esophageal sphincter as well. Interestingly, the chemical structure of theophylline is similar to that of caffeine which is another trigger of GERD.
It is a double edge sword because not only can acid reflux exacerbate one’s asthma, but asthma can also make one’s acid reflux worse. In addition to asthma making one’s acid reflux worse, there are several risk factors that may contribute to GERD and some of them may include obesity, alcohol use, diabetes mellitus, pregnancy, smoking, connective tissue diseases (e.g., systemic sclerosis or scleroderma), eating large meals, eating before bed, hiatal hernia, certain medications [e.g., aspirin, nonsteroidal anti-inflammatory drugs (NSAIDs), oral corticosteroids, bronchodilators, calcium channel blockers], and/or certain foods [e.g., caffeine, spicy foods, .garlic, onions, fatty foods, acidic foods (soda, citrus fruits, tomatoes)].
The diagnosis of GERD can be made by performing a comprehensive history from the patient along with observing relief when taking anti-reflux medications such as antacids and/or acid-blocking medications. If there is no improvement in acid reflux symptoms, there are several procedures that can be performed in order to help establish the diagnosis of acid reflux disease. Some of these procedures include upper endoscopy with or without a biopsy, barium swallow, pH monitoring (checks the acidity in the stomach), and esophageal manometry (checks the function of the lower esophageal sphincter and esophagus).
The treatment of GERD is directed at reducing the risk factors mentioned above as well as prescribing acid-blocking medications and antacids. By treating the underlying GERD in patients who are asthmatic with associated GERD, the symptoms of asthma (i.e., chest tightness, coughing, wheezing, and shortness of breath) may also be reduced.
The board certified allergy doctors at Black & Kletz Allergy have been diagnosing and treating asthma for more than 50 years. We treat both pediatric and adult and patients. Black & Kletz Allergy has offices in Washington, DC, McLean, VA (Tysons Corner, VA), and Manassas, VA. All 3 of our offices have on-site parking. For further convenience, our Washington, DC and McLean, VA offices are Metro accessible. Our McLean office location offers a complementary shuttle that runs between our office and the Spring Hill metro station on the silver line. For an appointment, please call our office or alternatively, you can click Request an Appointment and we will respond within 24 hours by the next business day. If you suffer from asthma or allergies, we are here to help relieve or hopefully end these undesirable symptoms that have been so bothersome, so that you can enjoy a better quality of life. Black & Kletz Allergy is dedicated to providing the highest quality allergy care in a calm, compassionate, and professional environment.
Individuals with asthma probably are unaware of the association between asthma and acid reflux (GERD). It is estimated that approximately 75% of asthmatics have GERD of some degree. GERD or acid reflux occurs when there is a backflow of stomach contents into the esophagus (i.e., swallowing tube). In other words, the stomach contents travel in the wrong direction and enter the esophagus, instead of the small intestines. People with GERD often complain of “heartburn,” which in reality is a burning sensation in the chest and/or throat usually in conjunction with a sour or bitter taste in the mouth. In addition, some individuals may experience other symptoms which may include sore throat, burping, abdominal bloating, nausea, wheezing, coughing, and/or the sensation that something is caught in one’s throat. It is the wheezing and coughing symptoms that cam mimic asthma in individuals without asthma. In true asthmatics, acid reflux may actually worsen their asthma symptoms, not just mimic them.
In patients with asthma, the typical asthma symptoms of chest tightness, coughing, wheezing, and/or shortness of breath may be aggravated if the underlying acid reflux is not treated appropriately. The first way this may occur is via small amounts of acid irritating the airways (similar to a chemical burn) which may in turn cause asthma symptoms. The second method may involve the triggering of a reflex in the airways to become narrower in order to prevent more acid from entering the airways. It is this narrowing of the airways that causes an individual with asthma to cough, wheeze, cough, experience chest tightness and/or feel short of breath.
In addition to the 2 methods above, some asthma medications may decrease the lower esophageal sphincter pressure thus relaxing this muscle which in turn will increase the severity of GERD or acid reflux. Asthma medications in the bronchodilator family such as albuterol (i.e., Proventil, ProAir, Ventolin, AccuNeb), terbutaline (i.e., Brethaire, Brethine), vilanterol, ipatroprium (i.e., Atrovent), salmeterol (i.e., Serevent), formoterol, (i.e., Foradil), levalbuterol (i.e., Xopenex), and Tiotropium (i.e., Spiriva) fall into this category. There are also asthma medications that are combinations of two or more medications, one of which is a bronchodilator, which can therefore increase acid reflux disease. The names of some of these medications include Advair, Dulera, Breo Ellipta, Symbicort, Combivent, AIRSUPRA, Trelegy, and DuoNeb. Theophylline (i.e., Theo-Dur, Uniphyl, Slo-Bid, Theo-24), an older but still useful oral bronchodilator asthma medication, has also been associated with an increase in GERD in patients by causing the relaxation of the lower esophageal sphincter as well. Interestingly, the chemical structure of theophylline is similar to that of caffeine which is another trigger of GERD.
It is a double edge sword because not only can acid reflux exacerbate one’s asthma, but asthma can also make one’s acid reflux worse. In addition to asthma making one’s acid reflux worse, there are several risk factors that may contribute to GERD and some of them may include obesity, alcohol use, diabetes mellitus, pregnancy, smoking, connective tissue diseases (e.g., systemic sclerosis or scleroderma), eating large meals, eating before bed, hiatal hernia, certain medications [e.g., aspirin, nonsteroidal anti-inflammatory drugs (NSAIDs), oral corticosteroids, bronchodilators, calcium channel blockers], and/or certain foods [e.g., caffeine, spicy foods, .garlic, onions, fatty foods, acidic foods (soda, citrus fruits, tomatoes)].
The diagnosis of GERD can be made by performing a comprehensive history from the patient along with observing relief when taking anti-reflux medications such as antacids and/or acid-blocking medications. If there is no improvement in acid reflux symptoms, there are several procedures that can be performed in order to help establish the diagnosis of acid reflux disease. Some of these procedures include upper endoscopy with or without a biopsy, barium swallow, pH monitoring (checks the acidity in the stomach), and esophageal manometry (checks the function of the lower esophageal sphincter and esophagus).
The treatment of GERD is directed at reducing the risk factors mentioned above as well as prescribing acid-blocking medications and antacids. By treating the underlying GERD in patients who are asthmatic with associated GERD, the symptoms of asthma (i.e., chest tightness, coughing, wheezing, and shortness of breath) may also be reduced.
The board certified allergy doctors at Black & Kletz Allergy have been diagnosing and treating asthma for more than 50 years. We treat both pediatric and adult and patients. Black & Kletz Allergy has offices in Washington, DC, McLean, VA (Tysons Corner, VA), and Manassas, VA. All 3 of our offices have on-site parking. For further convenience, our Washington, DC and McLean, VA offices are Metro accessible. Our McLean office location offers a complementary shuttle that runs between our office and the Spring Hill metro station on the silver line. For an appointment, please call our office or alternatively, you can click Request an Appointment and we will respond within 24 hours by the next business day. If you suffer from asthma or allergies, we are here to help relieve or hopefully end these undesirable symptoms that have been so bothersome, so that you can enjoy a better quality of life. Black & Kletz Allergy is dedicated to providing the highest quality allergy care in a calm, compassionate, and professional environment.
Allergy Nasal Spray Prevents COVID?
September 26, 2025 | Black & Kletz Allergy
 SARS-CoV-2 is the name of the coronavirus that is responsible for the COVID-19 pandemic that caused millions of deaths worldwide. Although vaccination and established population immunity have substantially mitigated the severity of acute SARS-CoV-2 infections, the virus continues to infect millions of people resulting in a number of hospitalizations and deaths. There is a need for an effective pre-exposure prophylaxis for the general population, particularly for high-risk groups such as individuals with preexisting conditions and the elderly.
Azelastine is a second-generation antihistamine, which is commonly used as a nasal spray under the trade name Astelin or Astepro. It is used for and quite effective for the relief of perennial and seasonal allergic rhinitis (i.e., hay fever) symptoms such as sneezing, runny nose, nasal congestion, post-nasal drip, itchy nose, itchy throat, sinus congestion, sinus headaches, itchy eyes, puffy eyes, watery eyes, and/or redness of the eyes. Azelastine also has previously demonstrated anti-viral activity against respiratory viruses such as influenza (i.e., flu), respiratory syncytial virus (RSV), and some coronaviruses, in addition to its anti-allergic and anti-inflammatory properties.
Clinical trials have demonstrated that azelastine nasal spray reduces viral load in patients with confirmed SARS-CoV-2 infection, suggesting therapeutic efficacy in the acute treatment of COVID-19. These findings prompted researchers to study whether azelastine can also be used as a prophylaxis to prevent Covid-19 infection.
In a clinical trial published in the Journal of American Medical Association-Internal Medicine this month (September 2025), a team of investigators attempted to shed further light on this topic. They recruited 450 healthy volunteers who were 18 to 65 years of age with no signs of an acute infection for the study. Participants were randomly assigned 1:1 to receive azelastine 0.1% nasal spray or placebo 3 times a day for 56 days. SARS-CoV-2 rapid antigen testing (RAT) was conducted twice a week with positive results confirmed by polymerase chain reaction (PCR).
The primary end point was the number of polymerase chain reaction-confirmed SARS-CoV-2 infections during the study. The results of the trial revealed that the incidence of PCR-confirmed SARS-CoV-2 infection was significantly lower in the azelastine group compared with the placebo group. As secondary end points, azelastine demonstrated an increase in mean time to SARS-CoV-2 infection among infected individuals, a reduction of the overall number of PCR-confirmed symptomatic infections, and a lower incidence of PCR-confirmed rhinovirus infections. Note that rhinovirus is the most common cause of the common cold.
Adverse events were comparable between the groups, except for a bitter taste which was experienced by more individuals in the azelastine group compared to placebo. Azelastine nasal spray has long been known to cause a bitter taste in one’s mouth in patients using the nasal spray for the treatment of allergic rhinitis.
The nasal mucus membrane, as the primary site of viral entry and replication, plays a critical role in the pathogenesis of respiratory viral infections. The ability of locally applied and locally acting azelastine nasal spray to significantly reduce SARS-CoV-2 and overall upper respiratory tract infections underscores the efficacy of topical nasal interventions.
The findings of this randomized clinical trial suggest that azelastine nasal spray may reduce the incidence of respiratory infections caused by SARS-CoV-2. Although not studied yet, maybe other nasal antihistamines such as olopatadine (i.e., Patanase) may also reduce or prevent the incidence of respiratory infections caused by SARS-CoV-2.
The board certified allergy specialists at Black & Kletz Allergy have 3 convenient locations with on-site parking located in Washington, DC, McLean, VA (Tysons Corner, VA), and Manassas, VA. The Washington, DC and McLean, VA offices are Metro accessible and we offer a free shuttle that runs between the McLean, VA office and the Spring Hill metro station on the silver line. The allergy doctors at Black & Kletz Allergy are extremely knowledgeable regarding allergic rhinitis (i.e., hay fever) as well as non-allergic rhinitis. We are very experienced using azelastine for the treatment of allergic rhinitis. We diagnose and treat both adult and pediatric patients. In addition, we treat patients with food, insect sting, medication, and skin allergies, as well as asthma, sinus disease, eosinophilic esophagitis, and immunological disorders. To schedule an appointment, please call any of our offices or you may alternatively click Request an Appointment and we will respond within 24 hours by the next business day. We have been servicing the Washington, DC, Northern Virginia, and Maryland metropolitan area for more than 5 decades and we look forward to providing you with comprehensive state-of the-art allergy care in a welcoming and professional environment.
SARS-CoV-2 is the name of the coronavirus that is responsible for the COVID-19 pandemic that caused millions of deaths worldwide. Although vaccination and established population immunity have substantially mitigated the severity of acute SARS-CoV-2 infections, the virus continues to infect millions of people resulting in a number of hospitalizations and deaths. There is a need for an effective pre-exposure prophylaxis for the general population, particularly for high-risk groups such as individuals with preexisting conditions and the elderly.
Azelastine is a second-generation antihistamine, which is commonly used as a nasal spray under the trade name Astelin or Astepro. It is used for and quite effective for the relief of perennial and seasonal allergic rhinitis (i.e., hay fever) symptoms such as sneezing, runny nose, nasal congestion, post-nasal drip, itchy nose, itchy throat, sinus congestion, sinus headaches, itchy eyes, puffy eyes, watery eyes, and/or redness of the eyes. Azelastine also has previously demonstrated anti-viral activity against respiratory viruses such as influenza (i.e., flu), respiratory syncytial virus (RSV), and some coronaviruses, in addition to its anti-allergic and anti-inflammatory properties.
Clinical trials have demonstrated that azelastine nasal spray reduces viral load in patients with confirmed SARS-CoV-2 infection, suggesting therapeutic efficacy in the acute treatment of COVID-19. These findings prompted researchers to study whether azelastine can also be used as a prophylaxis to prevent Covid-19 infection.
In a clinical trial published in the Journal of American Medical Association-Internal Medicine this month (September 2025), a team of investigators attempted to shed further light on this topic. They recruited 450 healthy volunteers who were 18 to 65 years of age with no signs of an acute infection for the study. Participants were randomly assigned 1:1 to receive azelastine 0.1% nasal spray or placebo 3 times a day for 56 days. SARS-CoV-2 rapid antigen testing (RAT) was conducted twice a week with positive results confirmed by polymerase chain reaction (PCR).
The primary end point was the number of polymerase chain reaction-confirmed SARS-CoV-2 infections during the study. The results of the trial revealed that the incidence of PCR-confirmed SARS-CoV-2 infection was significantly lower in the azelastine group compared with the placebo group. As secondary end points, azelastine demonstrated an increase in mean time to SARS-CoV-2 infection among infected individuals, a reduction of the overall number of PCR-confirmed symptomatic infections, and a lower incidence of PCR-confirmed rhinovirus infections. Note that rhinovirus is the most common cause of the common cold.
Adverse events were comparable between the groups, except for a bitter taste which was experienced by more individuals in the azelastine group compared to placebo. Azelastine nasal spray has long been known to cause a bitter taste in one’s mouth in patients using the nasal spray for the treatment of allergic rhinitis.
The nasal mucus membrane, as the primary site of viral entry and replication, plays a critical role in the pathogenesis of respiratory viral infections. The ability of locally applied and locally acting azelastine nasal spray to significantly reduce SARS-CoV-2 and overall upper respiratory tract infections underscores the efficacy of topical nasal interventions.
The findings of this randomized clinical trial suggest that azelastine nasal spray may reduce the incidence of respiratory infections caused by SARS-CoV-2. Although not studied yet, maybe other nasal antihistamines such as olopatadine (i.e., Patanase) may also reduce or prevent the incidence of respiratory infections caused by SARS-CoV-2.
The board certified allergy specialists at Black & Kletz Allergy have 3 convenient locations with on-site parking located in Washington, DC, McLean, VA (Tysons Corner, VA), and Manassas, VA. The Washington, DC and McLean, VA offices are Metro accessible and we offer a free shuttle that runs between the McLean, VA office and the Spring Hill metro station on the silver line. The allergy doctors at Black & Kletz Allergy are extremely knowledgeable regarding allergic rhinitis (i.e., hay fever) as well as non-allergic rhinitis. We are very experienced using azelastine for the treatment of allergic rhinitis. We diagnose and treat both adult and pediatric patients. In addition, we treat patients with food, insect sting, medication, and skin allergies, as well as asthma, sinus disease, eosinophilic esophagitis, and immunological disorders. To schedule an appointment, please call any of our offices or you may alternatively click Request an Appointment and we will respond within 24 hours by the next business day. We have been servicing the Washington, DC, Northern Virginia, and Maryland metropolitan area for more than 5 decades and we look forward to providing you with comprehensive state-of the-art allergy care in a welcoming and professional environment.
Autumn Allergies
September 26, 2025 | Black & Kletz Allergy
 Autumn has just begun and for some people it is a bittersweet moment. Although many individuals enjoy the lower temperatures that the autumn brings in the Washington, DC, Northern Virginia, and Maryland metropolitan region, many allergy sufferers are not so welcoming because with the lower temperatures, mold and weed pollen levels rise. The major weed culprit in the DC area is ragweed, although many other weed pollens increase during the Fall such as pigweed, sorrel, dock, milkweed, and lamb’s quarters. In the U.S., ragweed allergy is common as approximately 10% of the population suffers from ragweed pollen. Approximately 50% of all pollen-associated allergic rhinitis (i.e., hay fever) in North America is due to ragweed, particularly in the Eastern and Midwestern regions. Ragweed also causes allergic conjunctivitis (i.e., eye allergies) and asthma in other sensitive individuals.
There are 17 species of ragweed in North America. The only state in the U.S. without ragweed is Alaska. Each ragweed plant produces approximately 1 billion pollen grains. The ragweed plant lives only 1 season. Although ragweed is almost ubiquitous in the U.S., the pollen is even more widespread in rural areas. Ragweed is typically found along the side of the road, in vacant lots, in fields, and along riverbanks. Environmental factors play a role in the release of ragweed pollen. The warm weather in combination with the wind and increased humidity augments the release of ragweed pollen which in the mid-Atlantic region tends to begin in mid-August, peak in mid-September, and end with the first frost which typically occurs in late October. The ragweed pollen count tends to be its highest during the midday and lowest in the early mornings. The pollen released from the ragweed plant can travel hundreds of miles, like other pollens, so most of the U.S. population is exposed.
Most weed-sensitive individuals complain of the typical allergy symptoms associated with allergic rhinitis or allergic conjunctivitis. The symptoms may include sneezing, runny nose, nasal congestion, post-nasal drip, sinus congestion and pressure, sinus headaches, fatigue, itchy nose, itchy throat, snoring, itchy eyes, watery eyes, puffy eyes, and/or redness of the eyes. Individuals with asthma may also have an exacerbation of their asthma with symptoms such as wheezing, coughing, chest tightness, and/or shortness of breath. Patients prone to sinus infections (i.e., sinusitis) may develop a sinus infection during the autumn as a result of the high weed pollen count.
In addition to weeds, there are other environmental allergens that may be responsible for an uptick in allergy symptoms to allergic individuals in the autumn. Besides ragweed and other weeds, molds, dust mites, pets, and cockroaches are common allergens that may play a role in inducing allergic rhinitis, allergic conjunctivitis, and/or asthma symptoms in susceptible individuals. In fact, all of these allergens are perennial and may be bothersome to sensitive individuals throughout the year, not only in the autumn. In particular, leaf mold is quite annoying to many allergy sufferers, particularly to people who like to garden and rake leaves. It should be noted that dust mites, pets, and cockroaches are indoor allergens whereas weeds are outdoor allergens. Molds, however, are both indoor and outdoor allergens and are particularly bad in the Washington, DC metro area because DC was built on a swamp and there is high humidity in this region.
It is also interesting to note that there are certain foods that are closely associated with ragweed such that when eaten by ragweed-sensitive individuals, they may experience an itchy mouth, throat, and/or lips. People with this condition are said to have oral allergy syndrome or pollen-food allergy syndrome. It occurs in response to eating certain raw or uncooked fruits, vegetables, and/or nuts by the ragweed-sensitive person. Occasionally, one may experience itching of the hands when touching raw foods. Some examples of foods associated with ragweed pollen allergy include banana, melon (e.g., honeydew, cantaloupe, watermelon), chamomile tea, cucumber, zucchini, white potato, dandelion, sunflower seeds, and artichoke. Although ragweed-sensitive patients may develop an itchy mouth, throat, and/or lips when they eat these foods raw or fresh, when the fruit or vegetable is cooked or canned, the protein is denatured and thus destroyed which usually prevents the allergic reaction from occurring. In most cases, individuals are able to tolerate cooked and/or canned fruits and vegetables. Of note, oral allergy syndrome may also occur in individuals who have other pollen allergies such as tree pollen. A classic example of this is that some individuals who have birch tree allergies will have itching of the mouth, throat, and/or lips when eating raw or fresh pitted fruits (e.g., apples, peaches, plums, pears). Extra caution needs to be taken in cases where nuts cause symptoms because many individuals can have a classic nut allergy that is not associated with pollen which may be life-threatening. Such individuals should be prescribed a self-administered epinephrine device such as an EpiPen, Auvi-Q, Adrenaclick, or Neffy, an epinephrine containing nasal spray. Patients should be instructed that if they use the self-administered epinephrine device, they should go immediately to the closest emergency room.
The board certified allergists at Black & Kletz Allergy has 3 convenient locations in the Washington, DC metro area. We have offices in Washington, DC, McLean, VA (Tysons Corner, VA), and Manassas, VA which all offer on-site parking. The Washington, DC and McLean, VA locations are Metro accessible and there is a free shuttle that runs between the McLean, VA office and the Spring Hill metro station on the silver line. Please call us to make an appointment or you can click Request an Appointment and we will reply within 24 hours by the next business day. The allergy specialists of Black & Kletz Allergy are eager to help you with your autumn allergies (i.e., hay fever), asthma, hives, swelling episodes, mast cell condition, eosinophilic esophagitis, sinus issues, or any other allergy and immunology problems. We are dedicated to providing first-rate care to you as we have been doing in the Washington, DC metro area for more than 50 years.
Autumn has just begun and for some people it is a bittersweet moment. Although many individuals enjoy the lower temperatures that the autumn brings in the Washington, DC, Northern Virginia, and Maryland metropolitan region, many allergy sufferers are not so welcoming because with the lower temperatures, mold and weed pollen levels rise. The major weed culprit in the DC area is ragweed, although many other weed pollens increase during the Fall such as pigweed, sorrel, dock, milkweed, and lamb’s quarters. In the U.S., ragweed allergy is common as approximately 10% of the population suffers from ragweed pollen. Approximately 50% of all pollen-associated allergic rhinitis (i.e., hay fever) in North America is due to ragweed, particularly in the Eastern and Midwestern regions. Ragweed also causes allergic conjunctivitis (i.e., eye allergies) and asthma in other sensitive individuals.
There are 17 species of ragweed in North America. The only state in the U.S. without ragweed is Alaska. Each ragweed plant produces approximately 1 billion pollen grains. The ragweed plant lives only 1 season. Although ragweed is almost ubiquitous in the U.S., the pollen is even more widespread in rural areas. Ragweed is typically found along the side of the road, in vacant lots, in fields, and along riverbanks. Environmental factors play a role in the release of ragweed pollen. The warm weather in combination with the wind and increased humidity augments the release of ragweed pollen which in the mid-Atlantic region tends to begin in mid-August, peak in mid-September, and end with the first frost which typically occurs in late October. The ragweed pollen count tends to be its highest during the midday and lowest in the early mornings. The pollen released from the ragweed plant can travel hundreds of miles, like other pollens, so most of the U.S. population is exposed.
Most weed-sensitive individuals complain of the typical allergy symptoms associated with allergic rhinitis or allergic conjunctivitis. The symptoms may include sneezing, runny nose, nasal congestion, post-nasal drip, sinus congestion and pressure, sinus headaches, fatigue, itchy nose, itchy throat, snoring, itchy eyes, watery eyes, puffy eyes, and/or redness of the eyes. Individuals with asthma may also have an exacerbation of their asthma with symptoms such as wheezing, coughing, chest tightness, and/or shortness of breath. Patients prone to sinus infections (i.e., sinusitis) may develop a sinus infection during the autumn as a result of the high weed pollen count.
In addition to weeds, there are other environmental allergens that may be responsible for an uptick in allergy symptoms to allergic individuals in the autumn. Besides ragweed and other weeds, molds, dust mites, pets, and cockroaches are common allergens that may play a role in inducing allergic rhinitis, allergic conjunctivitis, and/or asthma symptoms in susceptible individuals. In fact, all of these allergens are perennial and may be bothersome to sensitive individuals throughout the year, not only in the autumn. In particular, leaf mold is quite annoying to many allergy sufferers, particularly to people who like to garden and rake leaves. It should be noted that dust mites, pets, and cockroaches are indoor allergens whereas weeds are outdoor allergens. Molds, however, are both indoor and outdoor allergens and are particularly bad in the Washington, DC metro area because DC was built on a swamp and there is high humidity in this region.
It is also interesting to note that there are certain foods that are closely associated with ragweed such that when eaten by ragweed-sensitive individuals, they may experience an itchy mouth, throat, and/or lips. People with this condition are said to have oral allergy syndrome or pollen-food allergy syndrome. It occurs in response to eating certain raw or uncooked fruits, vegetables, and/or nuts by the ragweed-sensitive person. Occasionally, one may experience itching of the hands when touching raw foods. Some examples of foods associated with ragweed pollen allergy include banana, melon (e.g., honeydew, cantaloupe, watermelon), chamomile tea, cucumber, zucchini, white potato, dandelion, sunflower seeds, and artichoke. Although ragweed-sensitive patients may develop an itchy mouth, throat, and/or lips when they eat these foods raw or fresh, when the fruit or vegetable is cooked or canned, the protein is denatured and thus destroyed which usually prevents the allergic reaction from occurring. In most cases, individuals are able to tolerate cooked and/or canned fruits and vegetables. Of note, oral allergy syndrome may also occur in individuals who have other pollen allergies such as tree pollen. A classic example of this is that some individuals who have birch tree allergies will have itching of the mouth, throat, and/or lips when eating raw or fresh pitted fruits (e.g., apples, peaches, plums, pears). Extra caution needs to be taken in cases where nuts cause symptoms because many individuals can have a classic nut allergy that is not associated with pollen which may be life-threatening. Such individuals should be prescribed a self-administered epinephrine device such as an EpiPen, Auvi-Q, Adrenaclick, or Neffy, an epinephrine containing nasal spray. Patients should be instructed that if they use the self-administered epinephrine device, they should go immediately to the closest emergency room.
The board certified allergists at Black & Kletz Allergy has 3 convenient locations in the Washington, DC metro area. We have offices in Washington, DC, McLean, VA (Tysons Corner, VA), and Manassas, VA which all offer on-site parking. The Washington, DC and McLean, VA locations are Metro accessible and there is a free shuttle that runs between the McLean, VA office and the Spring Hill metro station on the silver line. Please call us to make an appointment or you can click Request an Appointment and we will reply within 24 hours by the next business day. The allergy specialists of Black & Kletz Allergy are eager to help you with your autumn allergies (i.e., hay fever), asthma, hives, swelling episodes, mast cell condition, eosinophilic esophagitis, sinus issues, or any other allergy and immunology problems. We are dedicated to providing first-rate care to you as we have been doing in the Washington, DC metro area for more than 50 years.
Alpha-Gal Syndrome Update
August 18, 2025 | Black & Kletz Allergy

 Over the last 10-15 years, the number of individuals with meat allergy has risen mainly because of a condition called alpha-gal syndrome or mammalian meat allergy. Alpha-gal syndrome was first identified by Dr. Thomas Platts-Mills at the University of Virginia School of Medicine in 2002. He discovered that the syndrome originated from the bite of lone star ticks (Amblyomma americanum). Specifically, the IgE antibody (i.e., the allergy antibody) response to the mammalian sugar molecule known as alpha-gal was associated with a delayed-onset swelling (i.e., angioedema) and/or anaphylaxis 2 to 8 hours after eating mammalian food products, such as beef, lamb, pork, and/or venison.
Alpha-gal, officially referred to as galactose-alpha-1,3-galactose, is a carbohydrate (i.e., sugar molecule) that exists in most mammals (e.g., pigs, cows, deer, sheep, rabbits, whales). It is not found however in humans or non-mammals such as birds, reptiles or fish. Lone star ticks can transfer this alpha-gal carbohydrate molecule to humans by first biting and feeding on mammals and then biting humans. After someone is bitten by a lone star tick, the alpha-gal molecule, which is present in the tick’s saliva, is transmitted into the individual’s bloodstream. In turn, that person will produce IgE antibodies as a defense mechanism against this foreign carbohydrate molecule (i.e., sugar molecule). As a result, that person now has alpha-gal IgE antibodies present in their bloodstream. Going forward, after the sensitization to alpha-gal occurs, whenever that individual eats mammalian meat which naturally contains the sugar molecule galactose alpha-1,3-galactose (i.e., alpha-gal), their alpha-gal IgE antibodies will bind and react against the alpha-gal present in the mammalian meat (e.g., pork, beef, venison, lamb, rabbit, whale) and cause the person to exhibit allergic symptoms. The typical and more common symptoms experienced by someone with alpha-gal syndrome include hives (i.e., urticaria), swelling (i.e., angioedema) and/or anaphylaxis
Over the last 10-15 years, the number of individuals with meat allergy has risen mainly because of a condition called alpha-gal syndrome or mammalian meat allergy. Alpha-gal syndrome was first identified by Dr. Thomas Platts-Mills at the University of Virginia School of Medicine in 2002. He discovered that the syndrome originated from the bite of lone star ticks (Amblyomma americanum). Specifically, the IgE antibody (i.e., the allergy antibody) response to the mammalian sugar molecule known as alpha-gal was associated with a delayed-onset swelling (i.e., angioedema) and/or anaphylaxis 2 to 8 hours after eating mammalian food products, such as beef, lamb, pork, and/or venison.
Alpha-gal, officially referred to as galactose-alpha-1,3-galactose, is a carbohydrate (i.e., sugar molecule) that exists in most mammals (e.g., pigs, cows, deer, sheep, rabbits, whales). It is not found however in humans or non-mammals such as birds, reptiles or fish. Lone star ticks can transfer this alpha-gal carbohydrate molecule to humans by first biting and feeding on mammals and then biting humans. After someone is bitten by a lone star tick, the alpha-gal molecule, which is present in the tick’s saliva, is transmitted into the individual’s bloodstream. In turn, that person will produce IgE antibodies as a defense mechanism against this foreign carbohydrate molecule (i.e., sugar molecule). As a result, that person now has alpha-gal IgE antibodies present in their bloodstream. Going forward, after the sensitization to alpha-gal occurs, whenever that individual eats mammalian meat which naturally contains the sugar molecule galactose alpha-1,3-galactose (i.e., alpha-gal), their alpha-gal IgE antibodies will bind and react against the alpha-gal present in the mammalian meat (e.g., pork, beef, venison, lamb, rabbit, whale) and cause the person to exhibit allergic symptoms. The typical and more common symptoms experienced by someone with alpha-gal syndrome include hives (i.e., urticaria), swelling (i.e., angioedema) and/or anaphylaxisToxic Epidermal Necrolysis
August 13, 2025 | Black & Kletz Allergy
 Toxic epidermal necrolysis (TEN) is a painful, life-threatening skin condition. It is associated with blistering and peeling of large areas of the skin, including mucous membranes such as the mouth, eyes, and/or genitals. If less than 10% of the body surface is involved, it is often called Stevens- Johnson syndrome (SJS).
In the more severe form, which is referred to as toxic epidermal necrolysis, usually more than 30% of the body surface area is affected. These 2 conditions overlap when between 10 and 30% of body surface is involved.
The most common cause of toxic epidermal necrolysis is an adverse reaction to medications. The most common medications implicated in the causation are antibiotics and anti-seizure medications.
Fortunately, toxic epidermal necrolysis/Stevens-Johnson syndrome is a very rare complication of medication use where it is estimated that there are 0.4 - 1.2 cases per million each year for toxic epidermal necrolysis and 1 - 2 cases per million each year for Stevens-Johnson syndrome. Certain genetic factors may predispose one to this condition. It can affect all age groups and is slightly more common in females than in males. Toxic epidermal necrolysis is 100 times more common in association with human immunodeficiency virus infection (HIV).
The most common medications causing toxic epidermal necrolysis are:
Toxic epidermal necrolysis (TEN) is a painful, life-threatening skin condition. It is associated with blistering and peeling of large areas of the skin, including mucous membranes such as the mouth, eyes, and/or genitals. If less than 10% of the body surface is involved, it is often called Stevens- Johnson syndrome (SJS).
In the more severe form, which is referred to as toxic epidermal necrolysis, usually more than 30% of the body surface area is affected. These 2 conditions overlap when between 10 and 30% of body surface is involved.
The most common cause of toxic epidermal necrolysis is an adverse reaction to medications. The most common medications implicated in the causation are antibiotics and anti-seizure medications.
Fortunately, toxic epidermal necrolysis/Stevens-Johnson syndrome is a very rare complication of medication use where it is estimated that there are 0.4 - 1.2 cases per million each year for toxic epidermal necrolysis and 1 - 2 cases per million each year for Stevens-Johnson syndrome. Certain genetic factors may predispose one to this condition. It can affect all age groups and is slightly more common in females than in males. Toxic epidermal necrolysis is 100 times more common in association with human immunodeficiency virus infection (HIV).
The most common medications causing toxic epidermal necrolysis are:
- Sulfonamides: Bactrim (i.e., sulfamethoxazole + trimethoprim)
- Beta-lactam Antibiotics: penicillins, cephalosporins
- Anticonvulsants: Lamictal (i.e., lamotrigine), Tegretol (i.e., carbamazepine, Dilantin (i.e., phenytoin), phenobarbital
- Allopurinol - A medication to lower high uric acid levels)
- Tylenol (i.e., acetaminophen or paracetamol)
- Viramune (i.e., nevirapine) - An anti-HIV medication
- Nonsteroidal Anti-Inflammatory Drugs (NSAIDs)
- Anti-cancer Medications
- Eyes - red, soreness, stickiness, photosensitivity
- Lips/Mouth - red crusty lips, painful mouth ulcers
- Pharynx/Esophagus - difficulty eating
- Genital Area and Urinary Tract - erosions, ulcers
- Upper Respiratory Tract - cough, respiratory distress
- Gastrointestinal Tract - diarrhea
- Dehydration and/or acute malnutrition
- Infection of the skin, mucous membranes, lungs (i.e., pneumonia), septicemia (i.e., blood poisoning) with bacteria or fungus
- Acute respiratory distress syndrome
- Gastrointestinal ulceration and/or perforation
- Shock and multiple organ failure
- Excessive blood clotting and/or bleeding
- Immediate discontinuation of the suspected triggering drug
- Nutritional and fluid replacement
- Temperature maintenance
- Pain relief
- Sterile handling and reverse isolation
- Systemic antibiotics at the first sign of an infection
- Topical antiseptics such as silver nitrate and chlorhexidine
- Dressings such as gauze with petrolatum (i.e., petroleum jelly)
- Frequent eye drops/ointments (e.g., antiseptics, antibiotic, corticosteroids)
- Mouthwashes and oral anesthetics
- Corticosteroids and immunosuppressant medications
- Immune globulin supplementation
Update on Pet Allergies
July 18, 2025 | Black & Kletz Allergy
 Pet allergies are quite common in the United States. About 20% of the U.S. population have cat allergies and 10% of the people in the U.S. have dog allergies. Approximately 70% of the homes in the U.S. have at least 1 pet with dogs being the most common pet, followed by cats. The percentage of homes with pets is higher than in the past as more and more families are getting pets. The Labrador Retriever is the most common pet dog and the Ragdoll is the most common pet cat. There are roughly 77 million pet dogs living in the U.S, however there are only 49 million households with dogs. In other words, each dog-owning home has an average of 1.5 dogs. In contrast, there are roughly 59 million cats living in approximately 32 million homes, which is equivalent to 1.8 cats per household.
When someone says that they are allergic to a pet, they are really saying that they are allergic to one or more of the proteins that are produced in the sebaceous glands of the skin (dander), saliva, and/or urine of that animal. In dogs, the major protein responsible for their allergies is called “Can f 1.” Note that the “Can” in “Can f 1” is short for canine or dog. This protein is produced by dogs and commonly found in the dog’s dander, saliva, and urine. In cats, similarly, the major proteins associated with their allergies are called “Fel d 1” and “Fel d 4.” Note that the “Fel” in both of the proteins “Fel d 1” and “Fel d 4” is short for feline or cat.
Besides the fact that there are different proteins responsible for allergies to cats and dogs, there is also a difference in the dander between these 2 pets. Cat dander is “sticky” whereas dog dander is not. Cat dander tends to stick to walls, clothing, upholstered furniture, bedding, and carpeting. It is commonly transported from one’s house to other houses or to workplaces. An example of this phenomenon is illustrated by the fact that if the Fel d 1 protein is measured on a cat owner’s clothing or upholstered furniture at work, it is likely that the protein will be found. In addition, it usually take several months for these “allergic” proteins to dissipate and become undetectable, despite a thorough cleaning of the home, due the stick-to-itiveness of cat dander. Although cat dander is stickier than dog dander, it does not mean that dog dander cannot stick to walls, clothing, upholstered furniture, bedding, and carpeting. It just means that the proteins associated with cat dander will linger more in one’s home more than dog dander. It should also be noted that since the proteins associated with cats are also found in urine, cat litter boxes are a rich source of these proteins and cat-allergic individuals should avoid exposure to litter boxes, if at all possible.
Interestingly, there is a condition called pork-cat syndrome that may affect individuals who are allergic to cats. In this syndrome, cat-allergic individuals develop an allergy to consuming pork. The severity can be severe and even life-threatening. Pork-cat syndrome affects females much more than male individuals since approximately 80% of the people with this condition are females. Avoiding the consumption of pork is crucial in these individuals and patients with this syndrome should carry a self-injectable epinephrine device (e.g., EpiPen, Auvi-Q, Adrenaclick) or an epinephrine nasal spray (e.g., Neffy). If they use the epinephrine containing device, they should go immediately to the closest emergency room.
Another interesting fact is that there is an increased incidence of horse allergy in some individuals that have cat and/or dog allergies due to a common protein that is shared between all 3 animals. The dander is the most common way in which horses cause allergic symptoms in humans. The horse’s saliva, urine, and the fecal material dropped by horse mites are other ways that individuals are exposed to the allergenic proteins of horses. There are roughly 4 million households in the U.S. that own horses. Obviously, horses do not normally live in people’s homes, but they still cause allergic symptoms in many individuals. Recently, it has become fashionable to own miniature horses, which in some cases, do live in the homes of their owners. Living in a house with a horse in general is probably not the best idea, but for individuals with horse allergies, it is especially not a good idea.
In rodents, unlike cats and dogs, in addition to the allergenic protein being present in the urine, dander, and saliva, certain rodents such as mice contain allergenic proteins in their mouse fecal droppings. In birds, the protein responsible for their allergies is also present on their feathers, as well as urine, dander, saliva, and fecal droppings.
The symptoms of pet allergies are essentially the same as with any other environmental allergy (i.e., hay fever or allergic rhinitis) such as dust mites, pollens, and/or molds. The main difference is that sometimes when a pet licks or scratches someone, the pet-allergic individual may get an itchy rash where they are licked or scratched. Otherwise, the most common symptoms when a pet-allergic individual is in close proximity to a pet may include sneezing, runny nose, nasal congestion, sinus congestion, post-nasal drip, generalized itching, hives, itchy eyes, watery eyes, puffy eyes, and/or redness of the eyes. In patients with asthma, chest tightness, wheezing, coughing, and/or shortness of breath may occur.
The diagnosis of pet allergies begins with a comprehensive history and physical examination. Allergy testing by skin testing or occasionally blood testing is the standard procedure to further identify if someone is allergic to pets.
The treatment of pet allergies begins with avoidance. This is not always easy and most people have a close bond with their pets, which is understandable. Nevertheless, if the pet cannot be avoided, simple measures to decrease exposure to them may help remedy the situation such as keeping the pet out of the bedroom or washing the pet fairly frequently. Medications such as antihistamines, decongestants, corticosteroid nasal sprays, antihistamine nasal sprays, anticholinergic nasal sprays, leukotriene antagonists, and/or allergy eye drops may be beneficial in helping to relieve the annoying allergy symptoms. When all of the above have been taken into consideration, allergy immunotherapy (i.e., allergy shots, allergy injections, allergy desensitization, allergy hyposensitization) is a very effective treatment to alleviate and prevent pet allergy symptoms.
Black & Kletz Allergy has board certified allergy doctors in 3 convenient locations in the greater Washington, DC, Northern Virginia, and Maryland metro area. Our allergy specialists very familiar with your furry friends and can help you with your pet allergies. The allergists at Black & Kletz Allergy diagnose and treat both children and adults. We offer on-site parking in our Washington, DC, McLean, VA (Tysons Corner, VA), and Manassas, VA locations. The Washington, DC and McLean, VA offices are Metro accessible and the McLean office has a free shuttle that runs between the McLean office and the Spring Hill metro station on the silver line. If you are concerned that you or your child has a pet allergy or any other type of allergy or asthma, please call us to schedule an appointment. Alternatively, you can click Request an Appointment and we will respond within 24 hours by the next business day. Black & Kletz Allergy has been serving the needs of allergy and asthma sufferers in the Washington, DC metropolitan area for more than 50 years.
Pet allergies are quite common in the United States. About 20% of the U.S. population have cat allergies and 10% of the people in the U.S. have dog allergies. Approximately 70% of the homes in the U.S. have at least 1 pet with dogs being the most common pet, followed by cats. The percentage of homes with pets is higher than in the past as more and more families are getting pets. The Labrador Retriever is the most common pet dog and the Ragdoll is the most common pet cat. There are roughly 77 million pet dogs living in the U.S, however there are only 49 million households with dogs. In other words, each dog-owning home has an average of 1.5 dogs. In contrast, there are roughly 59 million cats living in approximately 32 million homes, which is equivalent to 1.8 cats per household.
When someone says that they are allergic to a pet, they are really saying that they are allergic to one or more of the proteins that are produced in the sebaceous glands of the skin (dander), saliva, and/or urine of that animal. In dogs, the major protein responsible for their allergies is called “Can f 1.” Note that the “Can” in “Can f 1” is short for canine or dog. This protein is produced by dogs and commonly found in the dog’s dander, saliva, and urine. In cats, similarly, the major proteins associated with their allergies are called “Fel d 1” and “Fel d 4.” Note that the “Fel” in both of the proteins “Fel d 1” and “Fel d 4” is short for feline or cat.
Besides the fact that there are different proteins responsible for allergies to cats and dogs, there is also a difference in the dander between these 2 pets. Cat dander is “sticky” whereas dog dander is not. Cat dander tends to stick to walls, clothing, upholstered furniture, bedding, and carpeting. It is commonly transported from one’s house to other houses or to workplaces. An example of this phenomenon is illustrated by the fact that if the Fel d 1 protein is measured on a cat owner’s clothing or upholstered furniture at work, it is likely that the protein will be found. In addition, it usually take several months for these “allergic” proteins to dissipate and become undetectable, despite a thorough cleaning of the home, due the stick-to-itiveness of cat dander. Although cat dander is stickier than dog dander, it does not mean that dog dander cannot stick to walls, clothing, upholstered furniture, bedding, and carpeting. It just means that the proteins associated with cat dander will linger more in one’s home more than dog dander. It should also be noted that since the proteins associated with cats are also found in urine, cat litter boxes are a rich source of these proteins and cat-allergic individuals should avoid exposure to litter boxes, if at all possible.
Interestingly, there is a condition called pork-cat syndrome that may affect individuals who are allergic to cats. In this syndrome, cat-allergic individuals develop an allergy to consuming pork. The severity can be severe and even life-threatening. Pork-cat syndrome affects females much more than male individuals since approximately 80% of the people with this condition are females. Avoiding the consumption of pork is crucial in these individuals and patients with this syndrome should carry a self-injectable epinephrine device (e.g., EpiPen, Auvi-Q, Adrenaclick) or an epinephrine nasal spray (e.g., Neffy). If they use the epinephrine containing device, they should go immediately to the closest emergency room.
Another interesting fact is that there is an increased incidence of horse allergy in some individuals that have cat and/or dog allergies due to a common protein that is shared between all 3 animals. The dander is the most common way in which horses cause allergic symptoms in humans. The horse’s saliva, urine, and the fecal material dropped by horse mites are other ways that individuals are exposed to the allergenic proteins of horses. There are roughly 4 million households in the U.S. that own horses. Obviously, horses do not normally live in people’s homes, but they still cause allergic symptoms in many individuals. Recently, it has become fashionable to own miniature horses, which in some cases, do live in the homes of their owners. Living in a house with a horse in general is probably not the best idea, but for individuals with horse allergies, it is especially not a good idea.
In rodents, unlike cats and dogs, in addition to the allergenic protein being present in the urine, dander, and saliva, certain rodents such as mice contain allergenic proteins in their mouse fecal droppings. In birds, the protein responsible for their allergies is also present on their feathers, as well as urine, dander, saliva, and fecal droppings.
The symptoms of pet allergies are essentially the same as with any other environmental allergy (i.e., hay fever or allergic rhinitis) such as dust mites, pollens, and/or molds. The main difference is that sometimes when a pet licks or scratches someone, the pet-allergic individual may get an itchy rash where they are licked or scratched. Otherwise, the most common symptoms when a pet-allergic individual is in close proximity to a pet may include sneezing, runny nose, nasal congestion, sinus congestion, post-nasal drip, generalized itching, hives, itchy eyes, watery eyes, puffy eyes, and/or redness of the eyes. In patients with asthma, chest tightness, wheezing, coughing, and/or shortness of breath may occur.
The diagnosis of pet allergies begins with a comprehensive history and physical examination. Allergy testing by skin testing or occasionally blood testing is the standard procedure to further identify if someone is allergic to pets.
The treatment of pet allergies begins with avoidance. This is not always easy and most people have a close bond with their pets, which is understandable. Nevertheless, if the pet cannot be avoided, simple measures to decrease exposure to them may help remedy the situation such as keeping the pet out of the bedroom or washing the pet fairly frequently. Medications such as antihistamines, decongestants, corticosteroid nasal sprays, antihistamine nasal sprays, anticholinergic nasal sprays, leukotriene antagonists, and/or allergy eye drops may be beneficial in helping to relieve the annoying allergy symptoms. When all of the above have been taken into consideration, allergy immunotherapy (i.e., allergy shots, allergy injections, allergy desensitization, allergy hyposensitization) is a very effective treatment to alleviate and prevent pet allergy symptoms.
Black & Kletz Allergy has board certified allergy doctors in 3 convenient locations in the greater Washington, DC, Northern Virginia, and Maryland metro area. Our allergy specialists very familiar with your furry friends and can help you with your pet allergies. The allergists at Black & Kletz Allergy diagnose and treat both children and adults. We offer on-site parking in our Washington, DC, McLean, VA (Tysons Corner, VA), and Manassas, VA locations. The Washington, DC and McLean, VA offices are Metro accessible and the McLean office has a free shuttle that runs between the McLean office and the Spring Hill metro station on the silver line. If you are concerned that you or your child has a pet allergy or any other type of allergy or asthma, please call us to schedule an appointment. Alternatively, you can click Request an Appointment and we will respond within 24 hours by the next business day. Black & Kletz Allergy has been serving the needs of allergy and asthma sufferers in the Washington, DC metropolitan area for more than 50 years.
Erythema Multiforme
July 8, 2025 | Black & Kletz Allergy
 Erythema Multiforme is a type of a rash on the skin, usually triggered by an infection or an adverse effect of a medication. The condition gets its name from the appearance of reddish skin lesions of various forms seen at the same time.
Erythema multiforme affects less than 1% of the population. It is most common in young adults (aged 20 - 40 years) with a modest predominance in males. Some people are genetically predisposed to develop erythema multiforme.
Causes:
Erythema Multiforme is a type of a rash on the skin, usually triggered by an infection or an adverse effect of a medication. The condition gets its name from the appearance of reddish skin lesions of various forms seen at the same time.
Erythema multiforme affects less than 1% of the population. It is most common in young adults (aged 20 - 40 years) with a modest predominance in males. Some people are genetically predisposed to develop erythema multiforme.
Causes:
Erythema multiforme is an immunological response to either an infection or a medication, manifested on the skin. The most common causative factors are:
- Infections - Herpes simplex virus (most common), mycoplasma, cytomegalovirus, Epstein-Barr virus, influenza virus, coronavirus, etc.
- Medications - Antibiotics (e.g., sulfonamides, tetracyclines, erythromycin), aspirin, nonsteroidal anti-inflammatory drugs (NSAIDs) (e.g., ibuprofen, naproxen), anti-seizure medications, and vaccines.
- Systemic disorders - Inflammatory bowel disease, hepatitis, lymphoma, leukemia, and solid organ tumors.
- After exposure to the trigger, there is usually a prodromal period when the individual may experience mild fever, cold like symptoms, sore throat, headache, fatigue, and/or achiness.
- A few days later, the typical skin lesions will erupt. These lesions may be in the form of red papules (i.e., small raised bumps), vesicles (i.e., blisters filled with clear fluid), ulcers (i.e., skin sores), etc.
- Characteristic lesions are ‘bulls-eye’ target lesions with a central dusky area, surrounded by a pale edematous area and a peripheral reddish ring, demarcating it from the surrounding normal skin.
- Atypical lesions are raised with poorly defined borders and/or fewer zones of color variation.
- Several lesions in different developmental stages may be seen at the same time.
- The skin lesions are usually symmetrical, begin at the periphery and spread centrally. The skin lesions usually have a predilection to extensor surfaces (i.e., outer side of the limbs)
- Skin lesions may be very itchy, painful, and/or swollen.
- Confirmation of the diagnosis may require a skin biopsy.
- Tests for infections, especially herpes simplex virus, are needed.
There are 2 types of erythema multiforme:
- Erythema multiforme minor - Mild form of the illness that only affects the skin and causes a rash.
- Erythema multiforme major - Most severe form of the condition which may be life-threatening because it causes large areas of the skin to blister and peel. This type affects the mucus membranes in the mouth, eyes, and genitals. Individuals usually have systemic symptoms such as fevers and/or joint pain.
- Most cases of erythema multiforme are mild and self-limiting and usually resolve spontaneously after a few days or weeks.
- Antihistamines and topical corticosteroid medications are helpful in relieving the itching and discomfort associated with more severe skin lesions.
- Antiseptic and local anesthetic mouthwashes may help relieve the pain and irritation associated with mucus membrane lesions inside the mouth.
- Antihistamine or anti-inflammatory eye drops can treat redness, burning, and/or excessive tearing of the eyes.
- Proper care of skin lesions such as avoidance of scratching will help prevent the spread of infections.
- Most severe cases may need a course of systemic corticosteroids such as oral prednisone.
- Recurrent cases are usually treated with 6 months or more of continuous oral antiviral medications such as acyclovir.
- General hand and respiratory hygiene is important in order to reduce the risk of contracting viral and bacterial infections.
- Avoiding medications that had previously caused adverse reactions in the past.
The board certified allergy specialists at Black & Kletz Allergy have 3 locations in the Washington, Northern Virginia, and Maryland metropolitan area. Our offices are located in Washington, DC, McLean, VA (Tysons Corner, VA), and Manassas, VA. All of our offices have on-site parking and the Washington, DC and McLean, VA offices are also Metro accessible. The McLean office has a complementary shuttle that runs between our office and the Spring Hill metro station on the silver line. The allergists at Black & Kletz Allergy diagnose and treat both pediatric and adult patients. To make an appointment, please call our office or you may click Request an Appointment and we will respond within 24 hours by the next business day. The allergy doctors at Black & Kletz Allergy have been helping patients with allergic skin rashes, hives (i.e., urticaria), eczema (i.e., atopic dermatitis), as well as other causes of allergies including hay fever (i.e., allergic rhinitis), asthma, sinus disease, food allergies, medication allergies, insect sting allergies, and immunological disorders for more than 50 years. If you suffer from an allergic skin rash or any other type of allergies it is our mission to improve your quality of life by minimizing or preventing your unwanted and annoying allergy symptoms.
Prurigo Nodularis
June 27, 2025 | Black & Kletz Allergy
 Prurigo nodularis is a chronic inflammatory skin disease where an extremely itchy rash in the form of firm bumps called nodules appears most commonly on the arms, legs, upper back, and/or abdomen. The rash is usually symmetrically distributed on both sides of the body. The itchiness, burning, and stinging sensation associated with prurigo nodularis is so severe that it often interferes with sleep and one’s psychological well-being.
The exact cause of prurigo nodularis is unknown, but altered function of the immune system and nerves in the skin is believed to be associated with heightened sensations of itchiness (i.e., pruritus) that leads to frequent scratching. Frequent scratching and picking of the skin are also thought to contribute to further lesion formation and thickening seen in the disease.
Prurigo nodularis can occur at any age but is more common in the elderly. When it occurs in younger patients, it is more likely to be associated with inflammatory skin diseases, usually eczema (i.e., atopic dermatitis). Prurogo nodularis is also more likely to manifest in patients with other underlying medical conditions that affect multiple body systems, such as cancer, diabetes, chronic kidney disease, and HIV infection. Prurigo nodularis is not hereditary or contagious.
The rash and itching can be episodic or continuous, lasting for several months in some individuals. It is typically worsened by sweat, heat, synthetic clothing, and/or stress. The rash can range in severity from just a few to several hundred lesions. The lesions can range in size from 0.2 inches to 0.8 inches wide and can appear as firm, dome-shaped papules, nodules, or plaques. Lesions can be flesh-colored, pink, red, brown, or black in color.
Diagnosis:
Prurigo nodularis is a chronic inflammatory skin disease where an extremely itchy rash in the form of firm bumps called nodules appears most commonly on the arms, legs, upper back, and/or abdomen. The rash is usually symmetrically distributed on both sides of the body. The itchiness, burning, and stinging sensation associated with prurigo nodularis is so severe that it often interferes with sleep and one’s psychological well-being.
The exact cause of prurigo nodularis is unknown, but altered function of the immune system and nerves in the skin is believed to be associated with heightened sensations of itchiness (i.e., pruritus) that leads to frequent scratching. Frequent scratching and picking of the skin are also thought to contribute to further lesion formation and thickening seen in the disease.
Prurigo nodularis can occur at any age but is more common in the elderly. When it occurs in younger patients, it is more likely to be associated with inflammatory skin diseases, usually eczema (i.e., atopic dermatitis). Prurogo nodularis is also more likely to manifest in patients with other underlying medical conditions that affect multiple body systems, such as cancer, diabetes, chronic kidney disease, and HIV infection. Prurigo nodularis is not hereditary or contagious.
The rash and itching can be episodic or continuous, lasting for several months in some individuals. It is typically worsened by sweat, heat, synthetic clothing, and/or stress. The rash can range in severity from just a few to several hundred lesions. The lesions can range in size from 0.2 inches to 0.8 inches wide and can appear as firm, dome-shaped papules, nodules, or plaques. Lesions can be flesh-colored, pink, red, brown, or black in color.
Diagnosis:
- The characteristic appearance and distribution of the lesions, the chronicity, and association with other systemic disorders provide clues to the diagnosis of prurigo nodularis.
- The confirmation of the diagnosis is established by biopsy of the skin lesions and examination of them under a microscope. It usually reveals thickening of different areas of the outermost layer of the skin (i.e., epidermis) with distinct changes (i.e., hyperkeratosis) to the skin protein keratin. The layer below the epidermis, referred to as the dermis, shows an increase in several inflammatory white blood cell types.
- Blood tests including a complete blood cell count (CBC), a comprehensive metabolic panel (CMP) that includes liver and kidney function tests, and a thyroid hormone panel may be beneficial for diagnosing an underlying systemic disease that may be contributing to the prurigo nodularis.
Treatment:
- Behavioral treatments for prurigo nodularis include ways to prevent scratching and dryness, such as keeping fingernails short, wearing long sleeves, wearing gloves, bandaging lesions, cleaning skin with gentle cleansers, keeping skin moisturized with non-irritating lotions, and avoiding warm environments to reduce sweating. Recommended anti-itch lotions include calamine, menthol, and camphor lotions.
- Moisturizers such as petroleum jelly, fragrance-free and ceramide-rich creams or ointments, and fragrance-free oatmeal or hyaluronic acid creams.
- Second generation oral antihistamines such as Zyrtec, Xyzal, Allegra, Claritin, or Clarinex taken on a regular basis. Many patients need 2 to 3 times the regular daily dose to get adequate relief from the severe itching and/or burning sensation that can be present in some individuals.
- Some patients respond better to first generation sedating antihistamines such as Palgic, Periactin, Atarax, or Benadryl.
- Topical medications such as corticosteroids (e.g., triamcinolone, fluocinonide, betamethasone, mometasone, clobetasol, fluticasone, desoxymetasone), calcineurin inhibitors (e.g., pimecrolimus, tacrolimus), capsaicin (the spicy ingredient in chili peppers), and vitamin D.
- Phototherapy: Exposing affected areas of the skin to specific wavelengths of ultraviolet (UV) light may help reduce the itchiness and inflammation of the skin.
- In 2022, dupilumab (i.e., Dupixent), an interleukin-4 receptor alpha antagonist, was approved by the U.S. Food and Drug Administration (FDA) to treat adults with prurigo nodularis. It is a subcutaneous (SQ) injection which can be self-administered under the skin every 2 weeks.
- In 2024, nemolizumab (i.e., Nemluvio), an interleukin-31 receptor antagonist, was approved by the FDA to treat adults with prurigo nodularis. It is a subcutaneous (SQ) injection administered every 4 weeks.
- Immunosuppressants such as cyclosporin, azathioprine, and methotrexate are reserved for the most resistant cases of prurigo nodularis because they affect more body systems and can have more serious side effects.
The board certified allergy specialists at Black & Kletz Allergy will promptly answer any questions you may have regarding prurigo nodularis or any other itching disorder. Our allergists have been diagnosing prurigo nodularis and other skin conditions in the Washington, DC, Northern Virginia, and Maryland metropolitan area for more than 50 years. We have 3 convenient locations in the DC metro area with offices in Washington, DC, McLean, VA (Tysons Corner, VA), and Manassas, VA. There is on-site parking at each location and both the Washington, DC and McLean, VA offices are Metro accessible. There is a free shuttle that runs between our McLean, VA office and the Spring Hill metro station on the silver line. To schedule an appointment, please call us at any one of our 3 locations. Alternatively, you can click Request an Appointment and we will respond within 24 hours by the next business day. Black & Kletz Allergy is dedicated in providing the most up-to-date diagnostic and treatment modalities in the field of allergy, asthma, and immunology.












